Every Team Member is Dedicated to Defeating MASH
Madrigal is a biopharmaceutical company focused on delivering novel therapeutics for MASH, a serious liver disease with high unmet medical need.
Kim, patient advocate
About Madrigal
A madrigal is a form of Renaissance choral music known for beautiful harmonies. At Madrigal Pharmaceuticals, our work focuses on restoring balance and harmony to the liver, an organ that performs over 500 different vital functions in the human body.
What began as a small team of researchers in Conshohocken, Pennsylvania, is now a fully integrated biopharmaceutical company with hundreds of employees in the United States and Europe. Madrigal has established a leading clinical development program in MASH.
Our team is united by a single purpose: to lead the fight against MASH.
Our Story
Leading the Fight Against MASH
Leading the Fight Against MASH
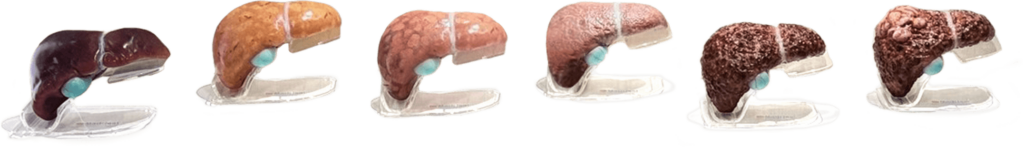
No family should have to go through the experience of watching a loved one struggle with decompensated cirrhosis or a liver transplant due to MASH. That’s why we aim to develop therapies that will help patients and the health system with this devastating disease.
Our Relentless Pursuit of Innovation
Madrigal has established a leading clinical development program in MASH and delivered the first treatment for people living with MASH in the U.S. and EU. Because of Madrigal’s success, MASH is no longer a ‘graveyard’ for drug innovation, and we continue to study our medication in additional populations with high unmet need.
Our Leaders
With extensive experience across science, business and healthcare, our diverse leadership team is shaping the future of MASH patient care.


Life at Madrigal
At Madrigal, we’re building more than treatments—we’re building a culture where people and innovation thrive.