Transforming Care for People with MASH
Our therapeutic approach directly targets the underlying causes of metabolic dysfunction-associated steatohepatitis (MASH), a serious liver disease.
Kerry, patient advocate
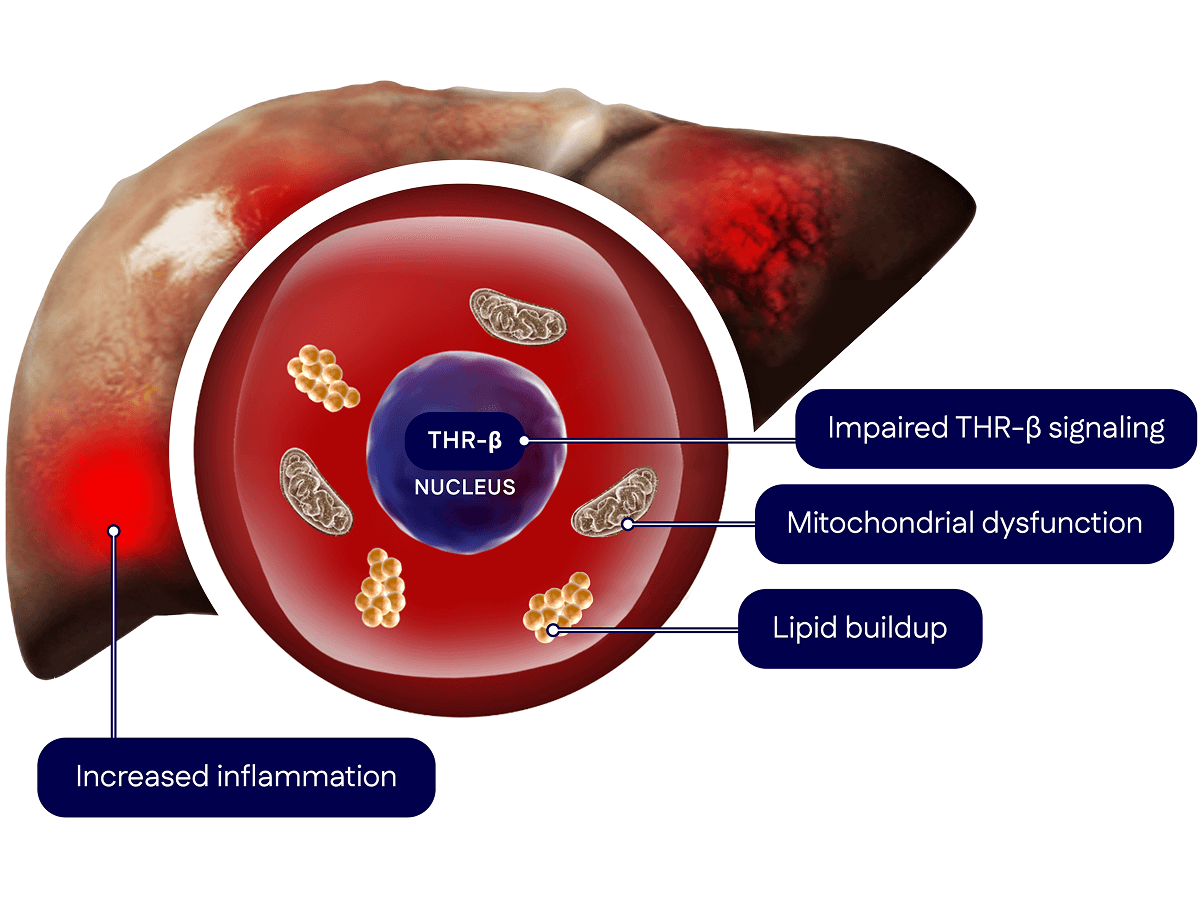
The Science Behind our Liver-Directed Therapy
Madrigal’s pioneering scientific advances in MASH emerged from more than a decade of research exploring the role of the thyroid hormone receptor-beta (THR-β) pathway in liver health.
In people with MASH, the liver’s ability to regulate key processes breaks down, leading to problems like inflammation, fat (lipid) buildup and reduced energy production (mitochondrial dysfunction). These issues damage liver cells, causing scarring (fibrosis) that can worsen over time.
Therefore, the goal of our scientific platform was to target THR-β directly in the liver to improve liver health and slow or reverse fibrosis.
“We’re committed to leading the way in
MASH R&D and building novel treatment paradigms
for patients with this serious disease.”– Rebecca Taub, M.D.
Madrigal Founder and Director
Research and Clinical Development
Madrigal has established the leading clinical development program in MASH, with multiple Phase 3 clinical trials:
Pivotal MAESTRO-
NASH Study
Patients with noncirrhotic MASH with moderate to advanced fibrosis (F2-F3)
MAESTRO-NAFLD-1 Safety Study
The first noninvasive Phase 3 study in MASLD (presumed MASH) (F1-F3)
MAESTRO-NASH Cirrhosis Study
An outcomes study in patients with well-compensated MASH cirrhosis (F4)
A Liver-Directed Therapy for MASH
Find out more about the first U.S. FDA-approved treatment for adults with MASH.
Advancing our MASH Pipeline
Madrigal is committed to building an industry-leading pipeline that will shape the future of MASH care.
← Scroll horizontally to view more →
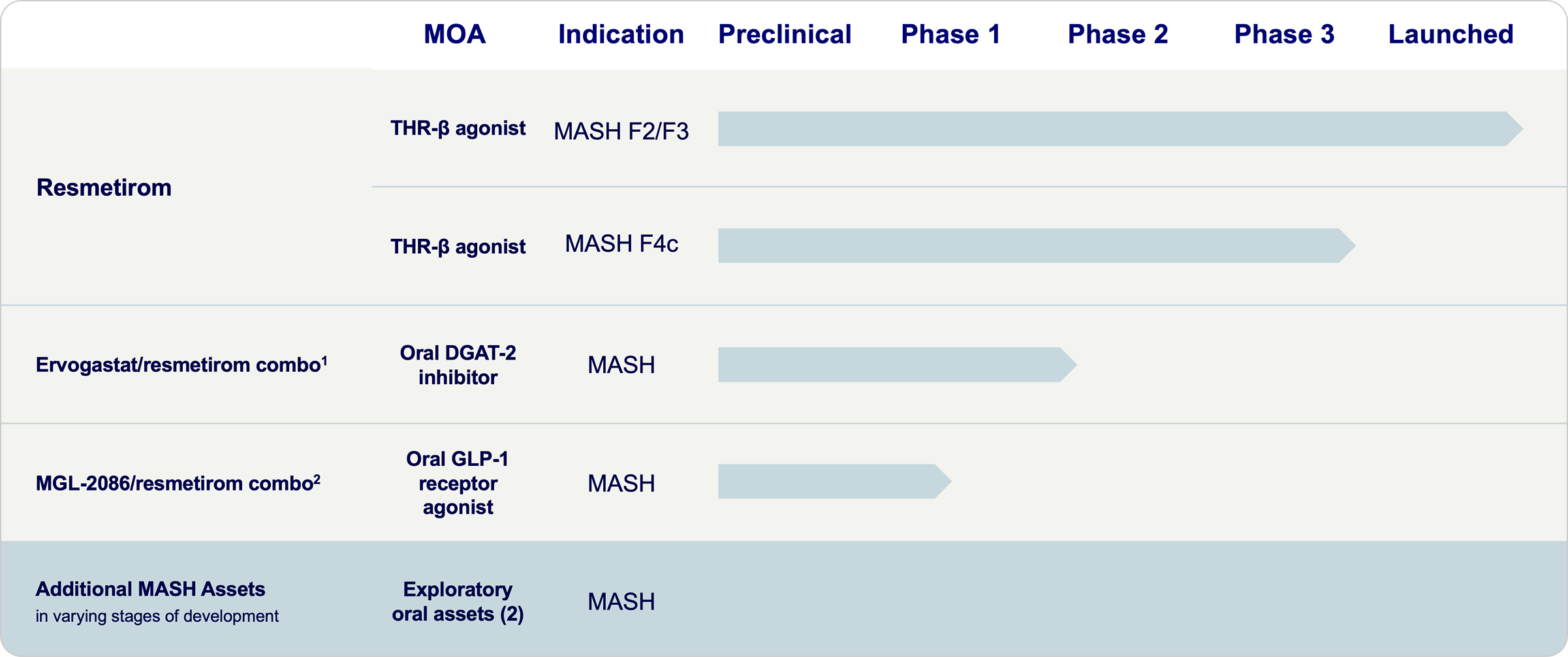
- Ervogastat monotherapy Phase 2b study completed by Pfizer; drug-drug (resmetirom/ervogastat) interaction (DDI) study expected to initiate in 2026; Phase 2 combination study expected to initiate in 2027 following regulatory discussions.
- MGL-2086 Phase 1 single ascending dose (SAD) study expected to initiate in 2Q26; MOA: mechanism of action.
References
Harrison SA, Bedossa P, Guy CD, et al; MAESTRO-NASH Investigators. A phase 3, randomized, controlled trial of resmetirom in NASH with liver fibrosis. N Engl J Med. 2024;390(6):497-509.
Karim G, Bansal MB. Resmetirom: an orally administered, small molecule, liver-directed, β-selective THR agonist for the treatment of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. touchREV Endocrinol. 2023;19(1):60-70.
Harrison SA, Ratziu V, Anstee QM, et al. Design of the phase 3 MAESTRO clinical program to evaluate resmetirom for the treatment of nonalcoholic steatohepatitis. Aliment Pharmacol Ther. 2024;59(1):51-63.